What does quality management mean?
Quality management is one of the central tasks of operational management and covers a wide range of measures for process planning, control and improvement. It is based on predetermined, defined requirements in order to ensure and continuously improve the quality of products and services and thus achieve greater customer satisfaction, among other things.
Quality management is an important tool in quality competition and is mandatory in certain industries, such as medical technology, food manufacturing, pharmaceutical manufacturing, healthcare, but also the automotive industry, aerospace and other industries with a strong focus on health and safety.
What is DIN EN ISO 9001 ff?
Ebbecke Verfahrenstechnik is certified according to DIN EN ISO 9001. DIN EN ISO 9001 ff. is a quality management standard that places specific requirements on a company’s management system in order to meet a certain standard in the implementation of quality management. At the end of a successful implementation process, a certificate is issued which documents the verification of certain standards to third parties.
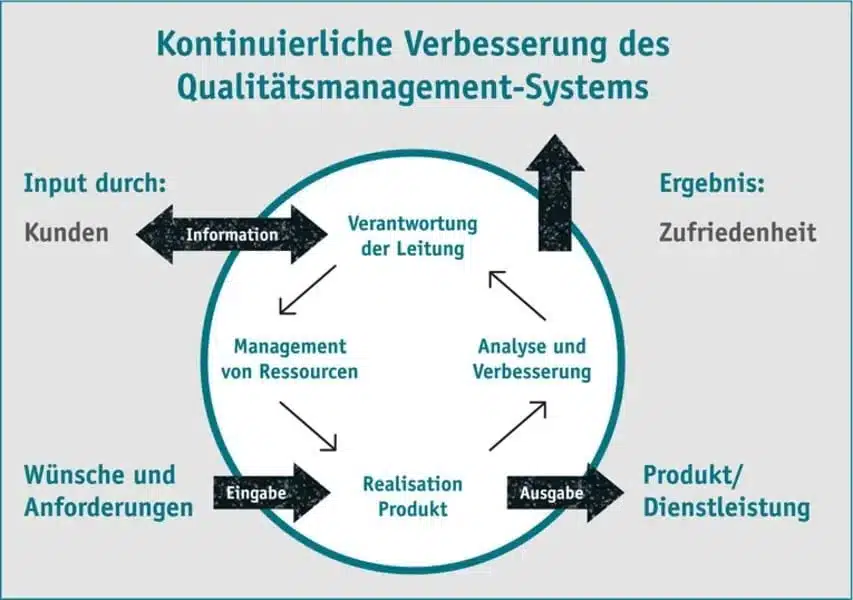
However, DIN EN ISO 9001 not only specifies the minimum requirements for a quality management system (QM system) that an organization must meet in order to satisfy customer expectations and official requirements with its products and services. At the same time, the management system is intended to stimulate a continuous improvement process and ensure that this is pursued on an ongoing basis.
With the introduction of a QM system, for example:
- increase the transparency of operational processes
- higher customer satisfaction can be achieved
- the error rate and thus costs are reduced
Principles of a QM system
QM systems are fundamentally process-oriented and highly customer-oriented. They are not an instrument limited to company management, but involve the various stakeholders from different areas of the company and hierarchical levels – also in terms of successful relationship management. Fact-based decision-making is at the forefront in all phases and areas of the quality management process.
The advantages of ISO 9001 certification
- The ISO 9001 certificate is an effective and confidence-building instrument for improving your own competitive position.
- QM certification in accordance with ISO 9001 gives the customer a feeling of trust and compliance with high quality standards.
- The regular review of an issued ISO 9001 certificate enables continuous quality assurance and improvement of quality management.
- A desired certification of the quality management system can be an additional motivation for the implementation of ISO 9001.
Legal significance
From a legal perspective, only ISO 9000 ff. and EN ISO 13485 for medical devices are accepted by all national standardization and certification bodies in the EU and, for the most part, worldwide. This is particularly important for internationally/globally operating companies, especially in matters of product liability. We are aware of this responsibility and are certified accordingly.
Quality management at Ebbecke Verfahrenstechnik
Every day, A. Ebbecke Verfahrenstechnik AG is guided by strictly defined quality standards and consistently adheres to the relevant guidelines. We do everything we can to reliably and continuously ensure the high quality of our products and services and work together with recognized, independent certification bodies to this end. The fact that our concept works is proven not only by our various certificates, but also by the high level of customer satisfaction. Find out more about quality management and the certifications of A. Ebbecke Verfahrenstechnik AG here.
Our ISO certifications:
https://www.ebbecke-verfahrenstechnik.de/unternehmen/qualitaetsmanagement
Online sources:
https://de.wikipedia.org/wiki/Qual%C3%A4tsplanung#Pr.C3.BCfplan, as of December 14, 2022, 08:50 UTC
https://de.wikipedia.org/wiki/ISO_9001, as of December 14, 2022, 08:55 UTC
https://de.wikipedia.org/wiki/Qualit%C3%A4tsmanagementnorm#EN_ISO_9001, as of December 14, 2022, 08:55 UTC